
The larger cations can then occupy the larger cubic holes made possible by the more open spacing. If the cations are too large to fit into the octahedral holes, the anions may adopt a more open structure, such as a simple cubic array. Depending on the relative sizes of the cations and anions, the cations of an ionic compound may occupy tetrahedral or octahedral holes, Relatively small cations occupy tetrahedral holes, and larger cations occupy octahedral holes. The larger type of hole is found at the center of six anions (three in one layer and three in an adjacent layer) located at the corners of an octahedron this is called an octahedral hole. The four anions surrounding this hole are arranged at the corners of a tetrahedron, so the hole is called a tetrahedral hole. The smaller of the holes is found between three anions in one plane and one anion in an adjacent plane. (As seen previously, additional electrons attracted to the same nucleus make anions larger and fewer electrons attracted to the same nucleus make cations smaller when compared to the atoms from which they are formed.) The smaller cations commonly occupy one of two types of holes (or interstices) remaining between the anions. In simple ionic structures, we usually find the anions, which are normally larger than the cations, arranged in a closest-packed array. As the positive charge increases on the cation it’s size decreases on contrary an increase in negative charge will increase the size of an anion, which inturn will affect the crystal structure. The size of the ion also depends upon the nature and magnitude of charge it possesses. Structures are determined by two principal factors: the relative sizes of the ions and the ratio of the numbers of positive and negative ions in the compound. Consequently, stable structures for ionic compounds result (1) when ions of one charge are surrounded by as many ions as possible of the opposite charge and (2) when the cations and anions are in contact with each other. Most monatomic ions behave as charged spheres, and their attraction for ions of opposite charge is the same in every direction. The packing of these ions into a crystal structure is more complex than the packing of metal atoms that are the same size. Ionic crystals consist of two or more different kinds of ions that usually have different sizes. Here, the anions are arranged in a face-centered cubic lattice with cations occupying the eight tetrahedral holes. The antifluorite structure is exhibited by compounds like sodium oxide, which has two cations and one anion. The unit cell has four calcium cations, each with a coordination number of eight, and eight fluoride anions, each with a coordination number of four. Here, the cations are arranged in a face-centered cubic lattice with the anions occupying all tetrahedral holes. Calcium fluoride, which has one cation and two anions, exhibits the fluorite structure.
.jpg)
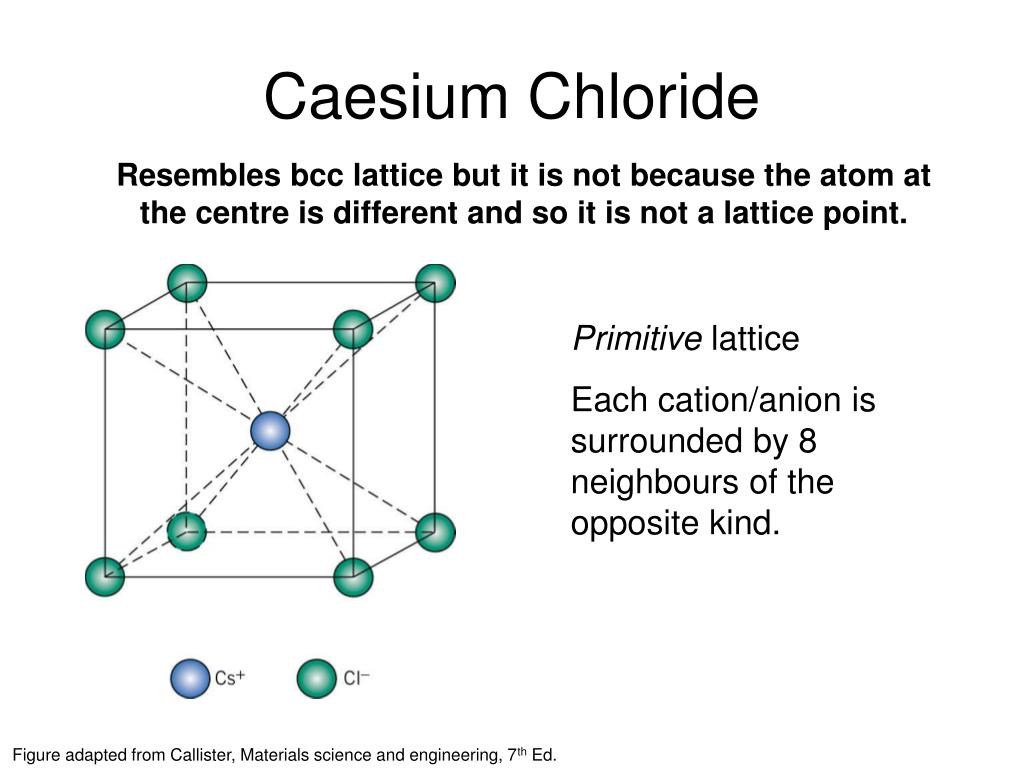
The proportion of cations to anions also affects which structure an ionic solid adopts. The unit cell has four sulfide anions and four zinc cations. Only half of all possible tetrahedral ‘holes’ are occupied by zinc cations, leaving the rest empty. Although the sulfide arrangement resembles a face-centered cubic lattice, the zinc cations are located within tetrahedra of sulfide anions, leading to a coordination number of four. Zinc sulfide has an even greater difference in ionic radii than that of sodium chloride, leading to the zinc blende structure. This is often referred to as the rock salt structure. The difference in ionic radii results in each sodium cation interacting with only six chloride anions and vice versa, with four chloride anions and four sodium cations assigned to the unit cell. Sodium chloride resembles a face-centered cubic lattice of chloride anions with the smaller sodium cations occupying the spaces between the anions. The caesium chloride unit cell is assigned one chloride anion and one caesium cation.Įach caesium ion is surrounded by eight chloride ions and vice versa, giving both caesium and chloride coordination numbers of eight. However, the charges and sizes of the ions greatly influence their packing configuration, leading to significant variation in crystal structure and coordination number.įor example, caesium chloride, where both ions are of similar sizes, resembles a primitive cubic lattice of chloride anions with caesium cations in the centers. Ionic solids typically are closely packed in a crystal lattice.


 0 kommentar(er)
0 kommentar(er)
